WTC Dust (911 Investigator 1.1): Unterschied zwischen den Versionen
Zur Navigation springen
Zur Suche springen
K |
K |
||
| Zeile 3: | Zeile 3: | ||
:from [[911 Investigator 1.1]] | :from [[911 Investigator 1.1]] | ||
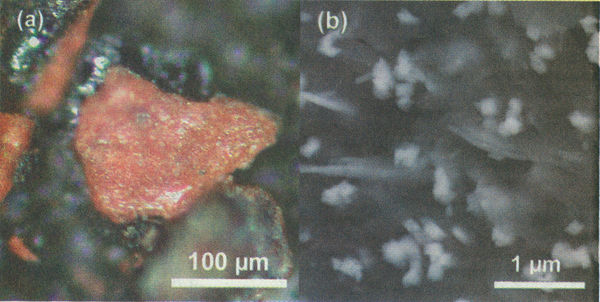
| − | [[Datei:Ae911 1 p22.jpg|600px|left|thumb|Highly energetic red/gray Chip discovered in the WTC dust at optical magnification (a) and 50, | + | [[Datei:Ae911 1 p22.jpg|600px|left|thumb|Highly energetic red/gray Chip discovered in the WTC dust at optical magnification (a) and 50,000X electron microscope magnification (b). The latter image shows consistently sized iron oxide particles approximately 100 nm across and 40 nm-thick wafers of aluminum embedded in a carbon-rich matrix]] |
{| class = "wikitable" | {| class = "wikitable" | ||
!What is Thermite? | !What is Thermite? | ||
| + | |- | ||
| | | | ||
Thermites are a class of compounds used for various purposes like welding, extraction of metals from ores, or, by the military, as an incendiary capable of damaging tanks and other equipment. The most common form of thermite is based on aluminum powder and iron oxide; the term "thermite" is therefore often used as a synonym for the aluminum/iron oxide thermite mixture. Thermite reactions are highly exothermic - i.e. they release relatively large amounts of energy. The common aluminum/iron oxide thermite mixture reacts into aluminum oxide, which is present in a whitish aerosol/"smoke", and iron, present as molten iron at temperatures of up to 4,500° F. | Thermites are a class of compounds used for various purposes like welding, extraction of metals from ores, or, by the military, as an incendiary capable of damaging tanks and other equipment. The most common form of thermite is based on aluminum powder and iron oxide; the term "thermite" is therefore often used as a synonym for the aluminum/iron oxide thermite mixture. Thermite reactions are highly exothermic - i.e. they release relatively large amounts of energy. The common aluminum/iron oxide thermite mixture reacts into aluminum oxide, which is present in a whitish aerosol/"smoke", and iron, present as molten iron at temperatures of up to 4,500° F. | ||
|} | |} | ||
Version vom 23. Januar 2016, 22:36 Uhr
- Advanced Pyrotechnic or Explosive Material Discovered in WTC Dust
- BY GREGG ROBERTS & ANDREA DREGER
- from 911 Investigator 1.1
| What is Thermite? |
|---|
|
Thermites are a class of compounds used for various purposes like welding, extraction of metals from ores, or, by the military, as an incendiary capable of damaging tanks and other equipment. The most common form of thermite is based on aluminum powder and iron oxide; the term "thermite" is therefore often used as a synonym for the aluminum/iron oxide thermite mixture. Thermite reactions are highly exothermic - i.e. they release relatively large amounts of energy. The common aluminum/iron oxide thermite mixture reacts into aluminum oxide, which is present in a whitish aerosol/"smoke", and iron, present as molten iron at temperatures of up to 4,500° F. |